Company Profile
Corporate Information
| Company Name | LEQUIO PHARMA CO.,LTD. |
|---|---|
| Location | [Headquarters] 16-3, Nishi 2-chome, Naha, Okinawa, Japan, 900-0036 (Map) Phone Number: +81-98-867-9114 Fax Number: +81-98-866-5844 E-mail:info@lequio-pha.co.jp |
| Established | February 28, 1991 |
| Capital | ¥100,000,000(As of December 1, 2021) |
| The end of the fiscal period | June |
| Representative | Chief Executive Officer Kinuko Oku |
| Business Activities | pharmaceuticals, research and development of health food products, and their manufacture and sales. |
| Relations | Name of Corporations |
|---|---|
| Joint development company for Zione Injection |
Mitsubishi Tanabe Pharma Corporation |
| Business partners | J-Dolph Pharmaceutical Co., Ltd. Cenway Technology Co., Ltd. |
| Corporate History | |
|---|---|
| February, 1991 | Establishes as Chuyakuken Co., Ltd. and headquarters opens in Matsuyama, Naha, Okinawa. |
| May, 1995 | Launches the joint development with Yoshitomi Pharmaceutical Industries, Ltd. (currently known as Mitsubishi Tanabe Pharma Corporation) for OC-108, a drug for internal hemorrhoids. |
| October, 1995 | Establishes Ginowan Research Laboratory in Ginowan, Okinawa. |
| March, 1999 | OC-108 gets approved by the Okinawa prefectural government under the Temporary Law concerning Measures for the Promotion of the Creative Business Activities of Small and Medium Enterprises. |
| April, 1999 | Joins the Osaka Pharmaceutical Manufacturers Association (currently known as Kansai Pharmaceutical Industries Association) as a full-fledged member. |
| September, 1999 | Completes Phase II clinical trial for OC-108. |
| April, 2000 | Renames company as Lequio Pharma Co., Ltd. |
| August, 2000 | Starts Phase III clinical trial for OC-108. |
| November, 2002 | Completes Phase III clinical trial for OC-108. |
| March, 2003 | Decides Zione Injection as sales name for OC-108. |
| March, 2003 | Files an application with the Ministry of Health, Labor and Welfare for the manufacturing approval of Zione (OC-108) as a new drug. (Applicant: Mitsubishi Pharma Corporation, currently known as Mitsubishi Tanabe Pharma Corporation) |
| July, 2004 | Obtains approval for manufacturing and marketing of new drug Zione Injection (OC-108) from the Ministry of Health, Labor and Welfare. (Obtainer: Mitsubishi Pharma Corporation curretly known as Mitsubishi Tanabe Pharma Corporation) |
| March, 2005 | Commences sales for Zione Injection. |
| February, 2006 | Jointly concludes a licensing agreement on Zione Injection with Yuhan Corporation (Seoul, Korea) with Mitsubishi Pharma Co., Ltd, now known as Mitsubishi Tanabe Pharma Co., Ltd.. |
| April, 2007 | Relocates the Ginowan Research Laboratory from Ginowan city to Uruma city. Renames it as Okinawa Research Laboratory. |
| June, 2007 | Receives the Okinawa Industrial Promotion Corporation’s grant, Okinawa Innovation Creating Project 2007, on the research project, “The pharmacological effect or function of the extract of Crassocephalum crepidioides and its development of functional ingredients.” |
| October, 2008 | Enters the health food industry. Commences the very first product, Lequio’s turmeric supplement GOLD |
| January, 2010 | Commences sales for the second health food product, Okinawan fermented papaya jelly bar LQ-001 |
| October, 2013 | Commences sales for the third health food product, Memorin. |
| April, 2020 | J-Dolph Pharmaceutical Co., Ltd. takes over the manufacturing and marketing approval of "Zione Injection" from Mitsubishi Tanabe Pharma Corporation. (Manufacturing and sales agency: J-Dolph Pharmaceutical Co., Ltd., partner: Lequio Pharma Co., Ltd.). |
| April, 2021 | Headquarters move in 16-3, Nishi 2-chome, Naha, Okinawa. |
| July, 2021 | Commences sales for the forth health food product, Athlin. |
| December, 2022 | Joins the Japan Anti-Aging Foundation. |
Message from The Management

Lequio Pharma originally started in February 1991 to obtain approval for the Chinese treatment of hemorrhoidal disease in Japan. While overcoming big obstacles as a new drug development venture company, we had Yoshitomi Pharmaceutical Industries, Ltd. (currently known as the Mitsubishi Tanabe Pharma Corporation) as a development partner, and we successfully launched the internal hemorrhoid theraputic agent, Zione Injection in March 2005. This was the very first drug approval given to the drug development venture company in Japan.
This new non-invasive therapy is recognized as effective as surgery, thus resulted as a new option in internal hemorrhoid therapy which brings less stress to the patient therefore contributing to their quality of life. Currently, it is used by over 664,000 patients at approximately 2,890 facilities nationwide.
It has always been my goal to produce new products of high value from Okinawa to the world. We believe that developing competitive products promotes the independence of Okinawan economy, create more employment which leads to further stages.
Although Okinawa has been at the mercy of the times, it used to be called the “Ryukyu Kingdom”, and as a small but independent nation without weapons, traded extensively with China and other Asian countries and flourished. It is our duty to live in the present, to constantly pursue and overtake the ideals our ancestors aimed for.
Since its foundation, we have been working on research for the prevention of dementia for the past 15 years.
It is said that this is a super-aging society for a long time, but the average life expectancy will continue to increase, and will greatly change the view of age. In order to respond to changing consciousness and values, we will respond to the health consciousness demanded by society, people and patients with products established by scientific evidence.
I would like to ask for your continued support in the years ahead.
January 2023
Chief Executive Officer
From Okinawa to the World. This is what Lequio Pharma aspires to achieve

Corporate Brand Lequio Pharma
From the 15th to the 16th centuries, Ryukyu Kingdom became recognized around the world as a place of overseas trade amongst Asian countries. New heights of prosperity was reached in the Ryukyu Kingdom that it was mentioned as “Lequio” in the account of his time in the East, written by Tomé Pires, a Portuguese who used to work at the Malacca Trading House. For the people in modern Okinawa, “Lequio” has become the symbolic name which carries the spirit of then flourishing Ryukyu Kingdom, rooted in strong sense of self-reliance with far-sighted innovation and its eagerness to take on new challenges.
We combine this word with “Pharmaceutical” to create our original brand Lequio Pharma. As a pharmaceutical company originated in the community of Okinawa, we aspire to inherit this frontier spirit of our predecessors to bring health as a key to open the ever-widening future.
Corporate Philosophy
We aim to contribute to people’s health and happiness through the production of pharmaceuticals for the improvement of patients' QOL and nutraceuticals for disease prevention.

Corporate History and Beyond
Road to Founding
Starting a New Industry in Okinawa
Our Chief Executive Officer, Ms. Oku grew up in Itoman City, Okinawa. Itoman city is known for producing many hard-working heroic female figures such as Toshiko Teruya, Kane Kinjyo, and Natsuko Kinjyo. Ms. Oku was under scholarly influence of the place while Okinawa was in the wake of the post war, rising to recover from U.S. military control. The daily necessities were mainly from the U.S. military and the local economic system was heavily relying on the income from American bases, she started to grow her keen desire to start an industry unique for Okinawa. She went to Taiwan to study language during her time at Ryukyu University, this was when she was inspired by their way of self-reliant life which became a turning point for her.
After she graduated from university, she worked on the import and sale of the subtropical plants such as mango, litchi, and palm trees, with the objective of enriching the natural environment of Okinawa and searching for key plants that would become industries in addition to sugar cane. Although she has continued this business for about three years, she realized that in order to make an industry out of agriculture, she needed vast agricultural land and funding, but she had to suspend everything due to marriage and childcare. After coming back, she opened a restaurant in Naha city to raise funds for securing farmland.
She started from scratch with no experience on import or restaurant businesses, but sales doubled in five years and the business was so successful that it was recognized as an excellent taxpayer by the prefectural government.
One day, her senior from university showed her the sample of Chinese medicine for hemorrhoids that she brought back from China. When she heard that it was for the treatment of internal hemorrhoids without surgery, she had an epiphany and thought “This is it!”.
The cost of logistics was the bottleneck of the Okinawan economy in the import business, but Ms. Oku saw the potential of this low-cost, competitive, and high value-added drugs. She flew to Beijing immediately to see the effects of the drug with her own eyes and confirmed her conviction. She managed to get the approval from the inventor, Professor Shi Zhao Qi, for developing the medicine in Japan and beyond. After returning to Japan, she was introduced to meet Professor Shuji Tsuchiya (Yokohama City University) and Professor Yukio Sumikoshi (then Social Insurance Central General Hospital, now known as Tokyo Yamate Medical Center).
Becoming Japan’s First Achievement
Working in the Lab
In 1991, Ms. Oku and her colleagues established Chuyakuken Co., Ltd., to research and develop the treatment for hemorrhoids.
Normally, it takes about 15 years to process all the necessary procedures, from basic research, non-clinical trials to clinical trials, before a drug is approved and actually commercialized. Whilst there were already some therapeutic drug developments for hemorrhoids in the process, the Zione brought back to Okinawa had the problem of sedimentation over time, making them less stable as Japanese medicine. Ms. Oku stayed persistent and asked for advice from a specialist to elucidate the sediment. At night she operated a restaurant while she herself collected data in the research laboratory. After many trials and errors, she managed to overcome obstacles through perseverance and determination, which was awarded with acquiring the patent in over 17 countries including Japan.
Further development was faced with challenges in securing the funding in a highly competitive process. In order to be approved as a drug, it must pass through a number of exams, including animal tests for safety, pharmacokinetics and pharmacology, and clinical trials. Animal experiments alone required tens of millions to hundreds of millions of yen per year. Ms. Oku reflects, “It’s because I had no knowledge on developing new drugs, I could embark on this journey. If I had known how hard it was, I wouldn’t have done it.”
Becoming Japan’s First Achievement
In order to get manufacturing approval by the Ministry of Health, Labor and Welfare, it was expected that it would be a time-intensive process, which needed to be performed with much bigger organizational capability with sufficient funding. After careful considerations, it was the right time to find the joint partnership that had the know-how to take on such bigger scale projects. Later, Yoshitomi Pharmaceutical Industries, Ltd. (currently known as Mitsubishi Tanabe Pharma Corporation) joined as a co-developer, and in 2004, we obtained the approval for the production and listing of the NHI price list of Zione Injection (an internal hemorrhoid sclerotherapy agent), with sales beginning in March 2005.
From this we established our company as the first drug discovery venture in Okinawa, as well as becoming the first venture business in Japan to introduce new drugs.
The New Medicine, Zione Injection
It is believed that one out of three Japanese people suffer from hemorrhoids today, but resection surgery has been the main treatment for severe hemorrhoids. Hemorrhoids developed inside the rectum are called internal hemorrhoids. Zione Injection is a drug that is injected into the affected area of internal hemorrhoids to sclerose and retract. Since it has significantly reduced pain after treatment and even can allow patients to return home on the day of treatment therefore resulting in less stress on the patients. This should contribute to the improvement of Quality of Life for patients. At present, Zione Injection has been introduced in many hospitals and is widely used as an internal hemorrhoid sclerotherapy and ALTA therapy. *1
*1 The acronym of ALTA has been taken from the two initial letters of the two active ingredients, aluminum potassium sulfate hydrate and tannic acid, which are the compounding agents for the local injection of Zione Injection.
| State of New Drug Development | |
|---|---|
| Product name / Development Code | Zione/OC-108 |
| Indication | Internal hemorrhoid |
| Stage | Filed on July 9th, 2004 (Application submitted by Mitsubishi Pharma Corporation) |
| Note | Joint development with Mitsubishi Pharma Corporation (Currently known as Mitsubishi Tanabe Pharma Corporation) |
Road Ahead
Looking for A New Drug
After succeeding on developing Zione Injection, it was time for something new. Ms. Oku focused on the traditional idea of “ishoku dogen” meaning that daily healthy meals can prevent and cure disease. In other words, “food is medicine”. With this in mind, she focused on medicinal quality of local plants such as turmeric and papaya for the idea of a new drug or other related products. First was turmeric. The active ingredient of turmeric is called curcumin, and they started research on its efficacy for prevention of dementia.
After entering into the health and food industry in 2008, the first product, Lequio’s Turmeric Supplement GOLD was launched. Prior to this, we held public seminars and introduced papers that curcumin is effective for reducing the risk of developing dementia, and conducted promotional activities. This later leads to the idea of the next product, Memorin.
In addition, in 2007, we received the Okinawa Industrial Promotion Corporation’s grant, “Okinawa Innovation Creating Project 2007” on the research project, “The pharmacological effect or function of the extract of Crassocephalum crepidioides and its development of functional ingredients.” In 2010, we launched the second product, Okinawa Fermented Papaya Jelly LQ-001, which helps to improve physical condition.

For Your Long-Lasting Health and Mind

In 2013, we launched the health supplement called Memorin as a result of research on dementia prevention. Dementia is a brain disorder in which cognitive functions such as judgment, comprehension, and adaptability are impaired due to memory impairment, making it difficult for one’s life. However, according to recent studies, it is possible to prevent dementia with appropriate measures. Even if you have dementia, as long as the right knowledge and information is shared with family, work and community, it is possible to live well with dementia. This was the reason why our Chief Executive Officer Ms. Oku started focusing on dementia because it was her wish for everyone to have long-lasting health as well as healthy minds.
The risk of longevity is dementia. This risk is higher in societies where life expectancy is high such as Okinawa, Japan. This is not a rare or special disease, but it can happen to anyone. Socially accepting dementia with better understanding and support is of course necessary, however, caretakers can also suffer from the same disease. For the course of long life, maintaining brain health as much as possible and proactively participating in society is now a social demand.
While the 100-year life awaits, it is our mission to support you for your enduring life!

Further Vision
I want to redefine the “elderly” and build a new culture that is based on this new understanding.
Today, the retired generation has a significant presence as a driving force in society. Even after they retire from work, many people still continue to stay very active in the society. What I would like to do is to change the conventional view of the “elderly” as socially vulnerable or beneficiaries of pensions, and create opportunities for them to make the most of their experience and skills as a senior who can still contribute to society. Most of them are still keen on enjoying their lives through actively participating in community with the Okinawan spirit of Namakara which means their life just only started.
If we could bring this new initiative to facilitate that each generations can have their hopes for their own future, and truly feel that long-lives can bring happiness, I think this can also bring solutions to the complex social issues that we have today.
As for our company, we have been working to create a new industry to enrich and be part of a solution to make Okinawa’s economy independent of the US military bases. We also provide support for the artistic and cultural activities such as the Okinawa-themed musical called Masary, produced in 1990. When we talk about a matured society, the relationship between economy, culture and arts are like the two wheels of the same vehicle, one cannot be separated for its own maturing processes. What we have in common with new drug development and musical production is the idea that “creating an industry and building a new culture based on its geography as well as its unique heritage is the only way we could bring true prosperity in Okinawa”.
It has always been my core theme to develop high value products and deliver them from Okinawa to the world, and this has never changed. We believe that creating competitive products will promote the independence of the Okinawan economy which would create more employment therefore leading to further stages.
Although Okinawa has been at the mercy of the times, it used to be known as the “Ryukyu Kingdom,” a small but independent nation without weapons, flourished by extensive trade with China and other Asian countries. We shall endeavor to follow and even overtake the visions that our ancestors aimed for.
What We Do
Our Research and Projects on Dementia
The risk of developing dementia among elderly people aged 65 and over is estimated to be 15%, and as of 2012, the Ministry of Health, Labor and Welfare estimated that about 4.62million people have dementia. Furthermore, the number of dementia patients is expected to exceed 7million in 2025, this is a major problem in our super-aging society.
Since 2008, Lequio Pharma has been conducting research on prevention and treatment of dementia using supplements containing nutrients derived from natural foods. There are some natural foods that have functional nutrients which can increase the level of the neurotransmitter acetylcholine in the brain, or some can reduce the accumulation of protein (beta-amyloid) in the brain which is the cause of Alzheimer’s disease.
In particular, curcumin contained in turmeric and Huperzine A in the fern plant Huperzia serrata have strong nutrient functions, and have great potential as therapeutic materials for prevention and treatment of dementia.
We carry out joint research with specialists on dementia and institutions such as pharmaceutical universities, and develop products based on scientific evidence.
It is our wish to contribute to your healthy life through our products.

Business Model
Establishing New Medicines and Health Foods Products Based on Unique Discoveries and Challenges
Lequio Pharma has been focusing on the long-practiced healing capacity of traditional medicine in Asian countries specifically Okinawa. Furthermore Lequio Pharma also focuses on the unknown healing properties of local plants and minerals that have yet to be discovered. Through our continuous research and development activities, and partnering with pharmaceutical companies, universities and other research institutes, we will develop products that contribute to the improvement of peoples’ QOL, preventing diseases therefore maintaining healthy lives.
Our Visions on Food Products
By utilizing the experience and know-how cultivated in the research and development of pharmaceuticals, we will identify the functionality of raw materials, develop and provide health food products based on solid scientific evidence. In particular, our turmeric research has a history of more than 20 years. Rich in minerals and curcumin, turmeric has been considered to be a very valuable plant in Okinawa even so that it was a monopoly good by the Ryukyu Dynasty. This is a prime example of our traditional idea ishoku dogen therefore “food is medicine”.
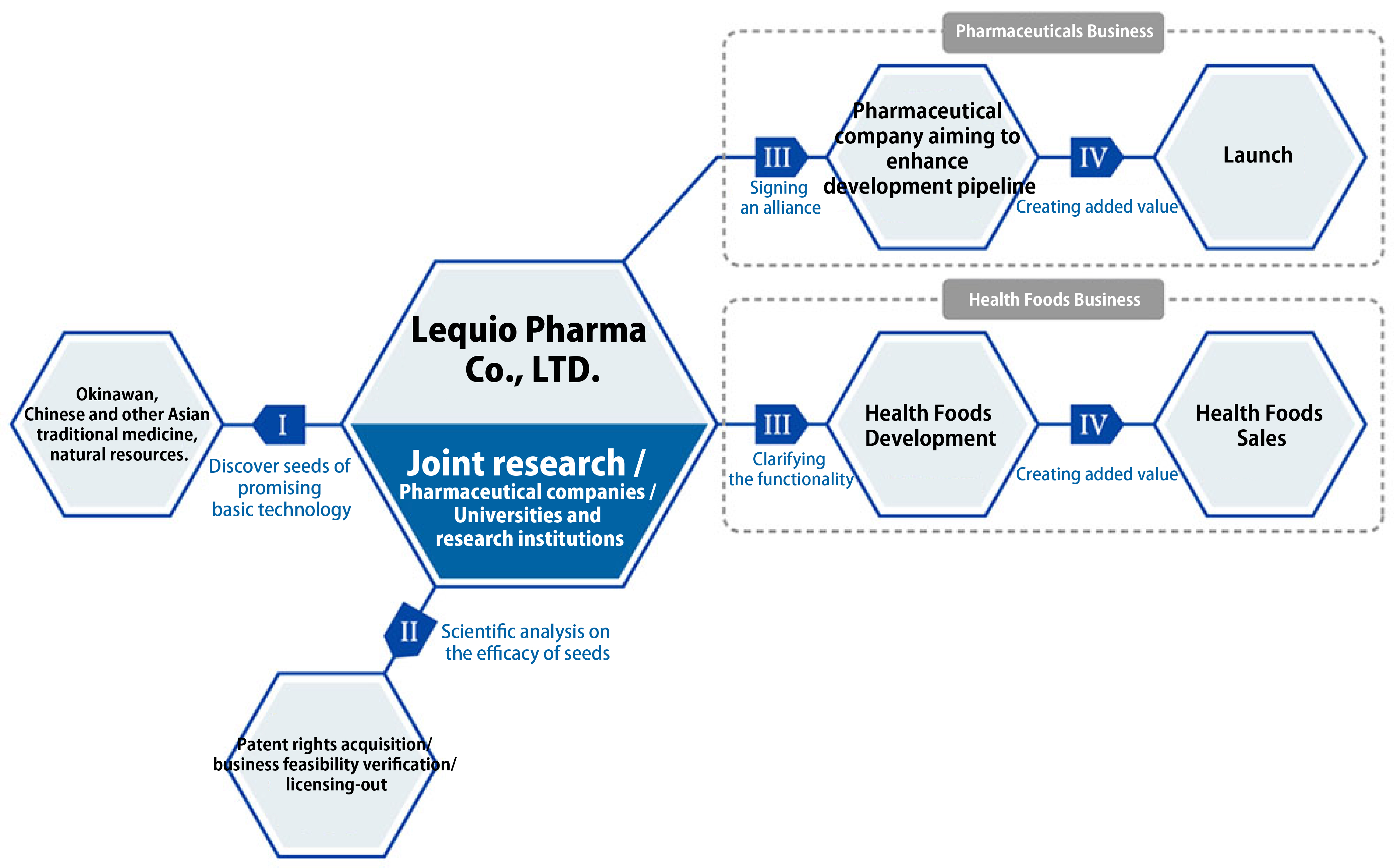
Business Process
I. We will discover seeds of promising basic technology.
II. We will conduct scientific analysis on the efficacy of seeds, as well as patent right acquisition and business feasibility verification.
[Pharmaceuticals Business]
III. We will sign business alliances with other pharmaceutical companies aiming to enhance development pipeline.
IV. We aim to create added value by launching products through joint development with alliance pharmaceutical companies.
[Health Foods Business]
III. We will develop health foods with excellent functionality.
IV. We will create added value by selling health foods with solid scientific evidence.
Our Product
Lequio Pharma re-examines the long-practiced healing capacity of traditional medicine from all over the world, and focuses on the still unknown healing properties of the natural world such as plants and minerals. From the discovery of new technologies developed by research institutions such as universities, we will strive to produce excellent products for a better society.
Cognitive Health Supplement, Memorin
Memorin is a supplement that protects your brain cognition, thinking and personality. Provides solutions for vibrant longevity.
Daily goals
- Take two Memorin capsules per day.
- Walk (aerobic exercise) fast for 20 minutes a day with smile.
- Be intellectually curious.
| Content | 60 Capsules (for 30 days) |
|---|---|
| Main Ingredients | Curcumin, Folic acid, Vitamin E, Vitamin B1, DHA, Vitamin B6, Vitamin C, Vitamin B2, Squalene, Vitamin B12, Huperzia serrata extract (Incl. Huperzine A)* |
| Supplement Facts | Per capsule (540mg)
Calories 3.14kcal |
| Suggested Usage | As a health supplement, take 2 capsules per day with water or warm water. |
*As a result of the reassesment of the food and drug classification, Huperzia serrata extract has been categorized as a pharmaceutical, therefore Memorin conatining it has not been available for sale since February 2023. Huperzia serrata extract free Memorin is available now.
Safety Warning
- Do not use this product if you are pregnant, breastfeeding, or may become pregnant.
- If you are taking other medication, please consult your doctor before use.
- Keep out of reach of children.
- Be careful not to choke on the capsule.
- Avoid direct sunlight, heat and humidity.

Hungover Care Supplemet, Lequio’s Turmeric GOLD
Our Lequio’s Turmeric GOLD has been patented in Japan for its high absorption and more bioavailability therefore curcumin is better delivered to your body.
| Content | 70 Capsules (for 35 days) |
|---|---|
| Main Ingredients (per capsule) |
Curcumin, Squalene, Piperine |
| Supplement Facts | Per capsule (420mg)
Calories 2.2kcal |
| Suggested Usage | As a health supplement, take 2 capsules per day with water or warm water. |
Safety Warning
- Do not use this product if you are pregnant, breastfeeding, or may become pregnant.
- If you are taking other medication, please consult your doctor before use.
- Keep out of reach of children.
- Be careful not to choke on the capsule.
- Avoid direct sunlight, heat and humidity, store in a cool, dry place.

Muscle Damage Care Suppliment, Athlin
Turmeric is not only good for your cognitive health, but it is a perfect remedy for muscle damage.
| Content | 60 Capsules / bag |
|---|---|
| Main Ingredients (per capsule) |
Curcumin, Bisbentiamine, Perilla oil, Squalene, Piperine |
| Supplement Facts | Per capsule (555mg)
Calories 3.34kcal |
| Suggested Usage | As a health supplement, take 3 capsules per day with water or warm water. |
Safety Warning
- If you are taking medication or regularly go to hospital, pregnant, breastfeeding, or may become pregnant, please consult your doctor before use.
- We take great care to check the quality of our products, but if you have received defected products, please contact us for a replacement.
- Be careful not to choke on the capsule.
- Avoid direct sunlight, heat and humidity, store in a cool, dry place.

